Polyvinyl alcohol (PVA) is a popular water-loving polymer membrane material. It has great use in food packaging, pervaporation, and wastewater treatment because it is chemically stable, resists acids and bases, forms films easily, and is safe to use. Its many hydroxyl groups give it good water-loving and antifouling traits. Still, these same groups cause two main problems: it's not very strong and doesn't hold up well in water. This means it can swell or even dissolve in water, which limits where it can be used.
To address these problems, scientists have tried changing PVA membranes by mixing it with other materials, forming nanocomposites, heating it, chemically crosslinking it, or using a mix of these ways .
1. Physical Modification: Boosting Function and Strength
Physical modification methods, like blending and nanocomposites, are popular because they are simple and easy to scale up for industrial production.
1.1 Blending Modification
Combining things to change PVA films involves mixing materials that work well and mix well with PVA to create the films. Chitosan (CS), for instance, is often used. The best part is that it gives PVA films good germ-killing abilities, greatly stopping or even killing Escherichia coli and Staphylococcus aureus. This helps Polyvinyl alcohol film (PVA film) be used in things like hemostatic dressings. However, the addition of blending materials can sometimes weaken the original mechanical properties of the PVA film, making the balance between functionality and mechanical strength a key challenge in this approach.
1.2 Nanocomposite Modification
Nanocomposite modification utilizes the unique surface-interfacial effects of nanosized fillers (such as nanosheets, nanorods, and nanotubes) to influence the internal structure of PVA films at the molecular level. Even with a small amount of filler, it can significantly improve the mechanical strength and water resistance of PVA films, while also expanding their electrical conductivity, thermal conductivity, and antimicrobial properties.
- Biopolymer nanomaterials: The addition of nanocellulose (CNC/CNF) and nanolignin (LNA) can improve the mechanical properties of PVA films because they are biocompatible and have good mechanical properties. It has been shown that intermolecular hydrogen bonding between these materials increases the tensile strength and flexibility of PVA films. Nanolignin, especially, does a great job at making PVA films stronger and more resistant to tearing. It also makes them better at blocking water vapor and UV light, which makes them more useful in food packaging.
- Carbon-based nanomaterials: Graphene, graphene oxide (GO), and carbon nanotubes (CNTs) possess exceptionally high mechanical strength and excellent electrical and thermal conductivity. GO can form multiple hydrogen bonds with PVA, enhancing both the film's mechanical strength and water resistance. For instance, adding bovine serum albumin to SiO₂ nanoparticles (creating SiO2@BSA) can more than double the tensile strength and elastic modulus of PVA films compared to using pure PVA films. Silicon-based nanomaterials: Silica nanoparticles (SiO2NPs) and montmorillonite (MMT) can effectively enhance the mechanical properties and thermal stability of PVA films. For example, SiO₂ NPs modified with bovine serum albumin (SiO2@BSA) can increase the tensile strength and elastic modulus of PVA films to more than double that of pure films.
- Metal and metal oxide nanoparticles: Silver nanoparticles (AgNPs) impart excellent electrical conductivity and antibacterial properties to PVA films; titanium dioxide nanoparticles (TiO2NPs) significantly enhance the photocatalytic activity of PVA films by reacting with hydroxyl groups on PVA molecular chains, showing great potential for wastewater treatment.
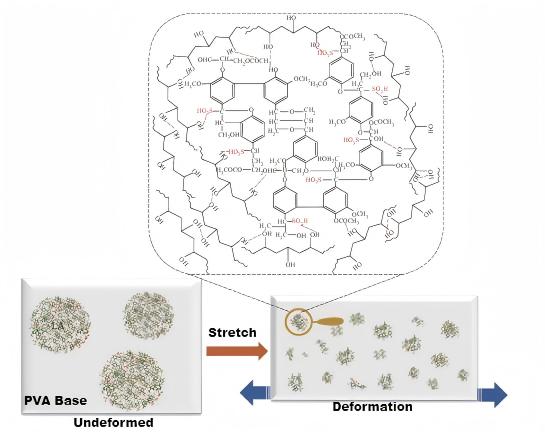
2. Chemical and Thermodynamic Approaches: Building a Stable Structure
2.1 Chemical Crosslinking Modification
Chemical crosslinking modification utilizes the numerous hydroxyl groups on PVA side chains to react with crosslinkers (such as dibasic/polybasic acids or anhydrides) to form a stable chemical bond (ester bond) crosslinking network between polymer chains. This method can more consistently improve the mechanical properties and water resistance of PVA film, significantly reducing its solubility in water and water swelling. For example, using glutaric acid as a crosslinker can simultaneously improve the tensile strength and elongation at break of PVA film.
2.2 Heat Treatment Modification
Heat treatment controls the movement of PVA molecular chains by adjusting temperature and time, optimizing the internal structure and increasing crystallinity.
- Annealing: Performed above the glass transition temperature, it increases the crystallinity of the PVA film, thereby enhancing its mechanical strength and water resistance.
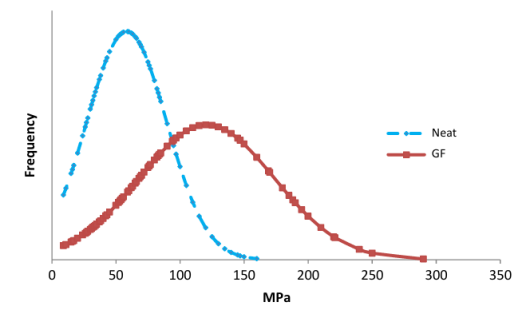
- Freeze-thaw cycling: Crystal nuclei are formed at low temperatures, and thawing promotes crystal growth. The resulting microcrystals serve as physical crosslinking points for the polymer chains, significantly improving the film's mechanical strength and water resistance. After multiple cycles, the tensile strength of PVA film can reach as high as 250 MPa.
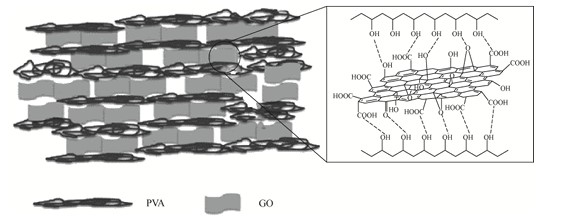
3. Synergistic Modification: Towards a High-Performance Future
A single modification method often fails to fully meet the complex performance requirements of PVA film in practical applications. It's tough to boost both strength and toughness at the same time. So, a key approach is to use two nanofillers or methods that work well together. This helps create PVA films that perform well in all areas. For example, combining chemical crosslinking with nanocomposites is currently one of the most promising strategies. Research has shown that synergistic modification of PVA films using succinic acid (SuA) as a crosslinker and bacterial cellulose nanowhiskers (BCNW) as a reinforcing filler significantly improves tensile strength and water resistance, effectively offsetting the shortcomings of single modification methods.
4. Conclusion and Outlook
Remarkable progress has been made in the modification of polyvinyl alcohol (PVA) films. Through the combined application of various strategies, including physical, chemical, and thermal treatments, the mechanical properties, water resistance, and multifunctionality of PVA films have been greatly enhanced. This has significantly promoted the practical application of modified PVA membranes in fields such as water treatment, food packaging, optoelectronic devices, and fuel cells.
Looking forward, research on modified PVA membranes (such as Modified PVA 728F) will focus on the following aspects:
- Synergistic modification: Further exploring the optimal synergistic effect of chemical crosslinking and nanocomposites to resolve the conflict between permeation flux and selectivity of membrane materials and achieve synergistic optimization of multiple properties.
- Functional Expansion: We plan to keep working on PVA films, giving them new features like self-healing and smart responses, so they can be used in more complicated situations.
By building on PVA's natural advantages and using advanced modification processes, polyvinyl alcohol films are likely to become even more widely used in the field of high-performance polymer materials.
Website: www.elephchem.com
Whatsapp: (+)86 13851435272
E-mail: admin@elephchem.com