With the development of nanotechnology, single crystal, polycrystalline, and amorphous nanomaterials have become research hotspots. These nanomaterials have different structures and properties, and have a wide range of applications. Dongguan Saite New Materials focuses on the production and sales of powder materials such as nano metal powder, oxide powder, carbide powder, alloy powder, etc., and has become a leader in the nano material market.
1、 Single crystal nanomaterials
Single crystal refers to the arrangement of grains in a material in the same direction. Single crystal nanomaterials are essential materials for preparing high-performance electronic components, conductor materials, and optical materials due to their high purity and complete crystal structure. For example, single crystal nano gold powder has a wide range of applications and can be used in conductive slurries, solar cell electrodes, biosensors, and so on.
2、 Polycrystalline nanomaterials
Polycrystalline grains are arranged together in different directions, and polycrystalline nanomaterials have excellent properties in strength, toughness, corrosion resistance, and can be used in fields such as electronics and aerospace materials. Polycrystalline nano titanium oxide powder can be used as a catalyst, UV barrier, anti UV coating, and other applications, while polycrystalline nano silicon carbide powder can be widely used in abrasive, cutting, ceramics and other fields.
3、 Amorphous nanomaterials
Amorphous nanomaterials refer to crystalline forms in materials that are not clearly defined. Amorphous nanomaterials have shown broad application potential in potential fields, such as preparing efficient catalysts, biomedical imaging materials, etc. Because amorphous nanomaterials exist in practical materials such as glass and have advantages such as transparency, hardness, and smooth surface, they are used in the production of high-tech products such as mobile phone screens and solar cells.
In the field of materials science, single crystal and polycrystalline are two different types of crystal structures. There are significant differences in their crystal structure, physical properties, preparation methods, and application fields.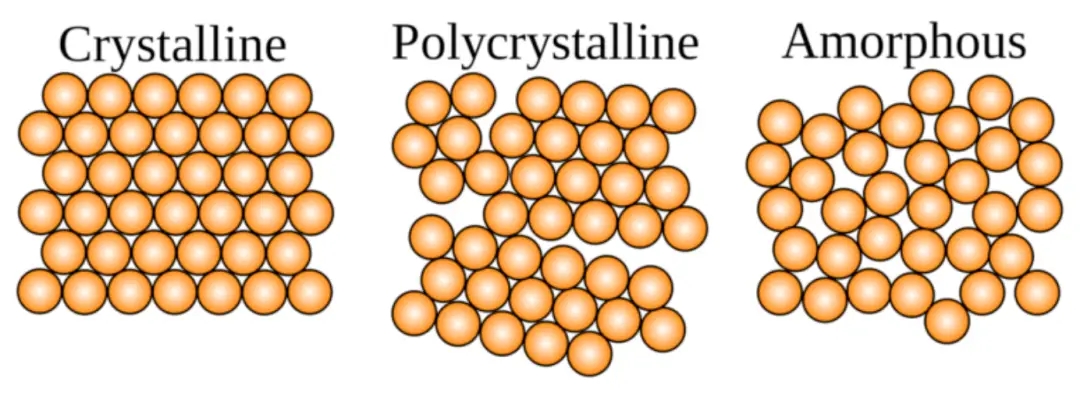
crystal structure
Single crystal refers to a crystal in which the lattice structure is complete, continuous, and orderly arranged, without grain boundaries or particle boundaries. This perfect crystalline structure enables single crystals to have higher crystallinity and crystal integrity. Polycrystalline materials are composed of many grains, which are connected to each other through grain boundaries. Grain boundaries are the interfaces between different grains within a crystal, where atoms or molecules are arranged relatively disorderly. Therefore, compared to single crystals, polycrystalline materials have a less perfect crystal structure, and the presence of grain boundaries also makes them heterogeneous and non-uniform.
physical property
Due to the highly ordered lattice structure of single crystals, their physical properties are usually more uniform and consistent than those of polycrystals. Single crystals exhibit good properties in electrical, optical, thermal, and mechanical fields, and possess isotropy (having the same properties in any direction). For example, monocrystalline silicon is widely used in semiconductor manufacturing for the production of integrated circuit chips due to its high conductivity, low conductor resistivity, and low leakage current. However, due to the presence of grain boundaries, polycrystalline silicon can lead to heterogeneity and non-uniformity in physical properties. For example, the resistivity and leakage current of polycrystalline silicon are usually higher than those of monocrystalline silicon.
Preparation method
The preparation process of single crystals usually requires precise control conditions and technical means, such as suspension method, vapor deposition method, floating zone method, etc. These methods can maintain a highly ordered lattice structure during the growth process of single crystals. The preparation of polycrystalline materials is relatively simple, often using methods such as melting and solidification. These methods can change the grain size and distribution by adjusting the preparation conditions, but the crystal structure of polycrystalline materials is often complex and difficult to control its lattice structure and growth direction.
application area
Due to its better physical properties and crystallinity, as well as the advantage of no grain boundaries, single crystals have a wide range of applications in certain specific fields. For example, in the field of optics, single crystal materials are used to produce high-precision optical lenses and laser devices. In addition, single crystal alloys are widely used in the aerospace industry to manufacture high-temperature structural components due to their high thermal stability and oxidation resistance. Polycrystalline materials, due to their simple preparation methods and low cost, are widely used in some basic material fields, such as steel manufacturing, ceramic manufacturing, etc.
In summary, there is a clear difference between single crystal and polycrystalline materials. Single crystals have a more perfect crystal structure and better physical properties, but their preparation is difficult and costly; The preparation of polycrystalline materials is relatively simple, but the crystal structure and physical properties are relatively complex and uneven. In terms of application fields, single crystals are widely used in some high-precision fields, while polycrystals have a wide range of applications in some basic materials fields.

Nanomaterials knowledge Home Nanomaterials knowledge Introduction to single crystal, polycrystalline, and amorphous nanomaterials
2024-05-13
Categories
Tags